Rise of Complex Technologies and a Challenging Reimbursement Landscape: How a Forward-Looking Indirect Treatment Comparison Feasibility Study Can Help Navigate Market Access
By Xcenda
HTA Quarterly | Summer 2019
Rise of Complex Technologies and a Challenging Reimbursement Landscape: How a Forward-Looking Indirect Treatment Comparison Feasibility Study Can Help Navigate Market Access
Date last updated: August 8, 2019
The Need
The reimbursement landscape is becoming increasingly complex. With the rise in technological innovation and evolving treatment pathways, there is even greater need for early collaboration and dialogue among industry, patients, and regulatory/reimbursement bodies. The future of health technology assessments (HTAs) is also uncertain with the current emphasis in Europe on harmonization of processes across European Union states calling for joint clinical assessments. This requires manufacturers to adapt to legislative changes and carefully consider appropriate comparators across markets. Given this context, this article discusses how an early, forward-looking indirect treatment comparison (ITC) feasibility study can help to overcome some of the challenges in the era of complex technologies and an uncertain HTA landscape to optimize patient access to safe and effective treatment.
What Is a Forward-Looking ITC Feasibility Study?
A traditional ITC feasibility study takes the current evidence-base of approved (ie, via the Food and Drug Administration [FDA] or the European Medicines Agency [EMA]), recommended (via recognized treatment guidelines), and/or commonly used comparators in a global landscape, to determine what can be analyzed via an ITC.
- Whether there are new connections to the existing ITC, allowing for greater indirect comparisons between products
- Whether restricted/targeted populations are being explored, calling into question the need for additional subgroup analyses, greater consideration of important treatment-effect modifiers, or more refined populations considered in any future trial
- Changes/trends in study designs over time to inform future drug development programs (eg, basket trials)
- Timeliness of the release of data and anticipated product approval/licensing on scope considerations in future reimbursement applications both within the ITC and economic models (where applicable)
What Can a Forward-Looking ITC Feasibility Study Inform?
A forward-looking ITC feasibility study can have various applications, as outlined in Table 1, which span throughout a product’s life cycle (Figure 1). In its early application, it can serve as a landscape assessment to understand unmet need; in phase 2/3, it can help to prepare for regulatory/reimbursement applications through, for example, early scientific advice and/or the generation of mock ITC results for input into an economic model.
Table 1. Ways a Forward-Looking ITC Feasibility Study Can Support a Product

Figure 1. Application of a Forward-Looking ITC Feasibility Study in a Product’s Life Cycle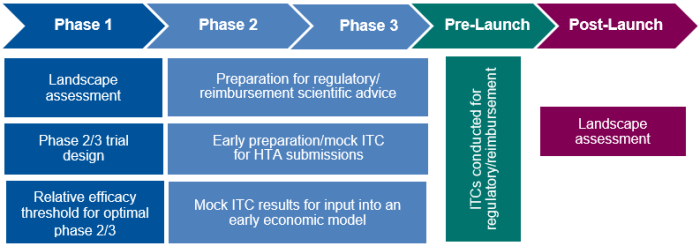
Conclusions
In the current rise of complex technologies and challenging reimbursement applications, there is even greater need for early preparation and considerations on how to overcome clinical and methodological hurdles to ensure that effective and safe products can be made available to patients. A forward-looking ITC is a tool to support the successful navigation of a product to the market. Once completed, it can be updated as needed, across the life cycle of a product, to inform various global health economics and outcomes research strategies on the road to regulatory/reimbursement approval and post-launch.
The article should be referenced as follows:
Rizzo M, Ballew N, Wissinger E. Rise of Complex Technologies and a Challenging Reimbursement Landscape: How a Forward-Looking Indirect Treatment Comparison Feasibility Study Can Help Navigate Market Access. HTA Quarterly. Summer 2019. August 27, 2019.
Sources:
- European Commission. 2018/0018 (COD): Proposal for a Regulation of the European Parliament and of the Council on Health Technology Assessment and Amending Directive 2011/24/EU. https://ec.europa.eu/health/sites/health/files/technology_assessment/docs/com2018_51_en.pdf. Accessed July 18, 2019.
- Love-Koh J, Peel A, Rejon-Parrilla JC, et al. The future of precision medicine: potential impacts for health technology assessment. Pharmacoeconomics. 2018;36(12):1439-1451.





