Only 17 months until the implementation of the new EU JCA—Latest developments and remaining uncertainties
By Xcenda
The clock is ticking down to the implementation of the EU JCA, yet many questions remain. What do we know, and how can we prepare for what comes next?

Previously in HTA Quarterly …
…we described the Joint Clinical Assessment (JCA) pilot phase and highlighted the uncertainties around the JCA process to come. Furthermore, we looked at the potential implications of the JCA on biopharma companies. In this article, we reflect on the evolution since our last article, latest insights, and what is still uncertain around the implementation of the JCA starting for oncology drugs and advanced therapy medicinal products (ATMPs) in 2025.
What do we know so far?
The EUnetHTA 21 Consortium will cease operations in September 2023 , after which continued collaboration will be facilitated under the European Union (EU) health technology assessment regulation (HTAR), with the Coordination Group on Health Technology Assessment (HTACG) supporting its implementation.
As the JCA/joint scientific consultations (JSCs) pilot phase organized by the EUnetHTA 21 consortium will come to an end this year, the penultimate Stakeholder Meeting was held on 12 May 2023 to prepare to transfer EUnetHTA 21 to the HTACG and their subfunctions. During the meeting, learnings from the JSCs and JCAs conducted during the pilot phase were discussed.
In total, 2 JCAs for medical devices were performed during the pilot phase. Interestingly, none was performed for pharma products because no applications were submitted. The EUnetHTA 21 conducted training exercises instead to test developed procedures and guidelines on compounds with positive Committee for Medicinal Products for Human Use (CHMP) opinion. Products that will be subject to the HTAR in 2025, such as oncology drugs and ATMPs, were selected and member states generated PICO (population, intervention, comparator, outcomes) criteria to simulate the scoping process which will be a crucial part of the EU JCA. The consolidated PICOs from the training exercises are due to be published during the summer and will provide important insights for the scoping process to be conducted starting in 2025. Based on these exercises, the Consortium concluded that the process of consolidating the PICOs provided by the individual countries was working well. Another learning was that national health technology assessment bodies (HTAbs) should be provided with more detailed background information on the disease area to further optimize the process and support national assessment bodies in defining the country-specific PICO criteria. As a next step, the Consortium proposed to test a process where nominated authors of the JCA report suggest PICOs for HTA bodies to approve.
To date (July 2023), 7 JSCs have been completed during the pilot phase. The key learnings from these pilot JSCs were that more open calls are needed, and further explanations of the prioritization criteria and better communication of the selection process should be provided to encourage applications. In July 2023, it was announced that scientific advice with the European Medicines Agency (EMA) and national HTA bodies—providing parallel scientific advice to support the planning of pivotal studies—will be led by the German Federal Joint Committee (G-BA) during the interim period from September 2023 until the end of 2024. The parallel EMA/HTAbs scientific advice coordinated by the G-BA during the interim phase will be a good opportunity for developers (referred to in official documents as health technology developers or HTDs) of eligible oncology drugs and ATMPs to seek regulatory and market access feedback to inform their clinical trial design and will provide important learnings for both biopharma companies and HTA bodies to optimally prepare for the upcoming EU JCA. Detailed guidance and application forms for the parallel scientific advice are available on the G-BA’s website.
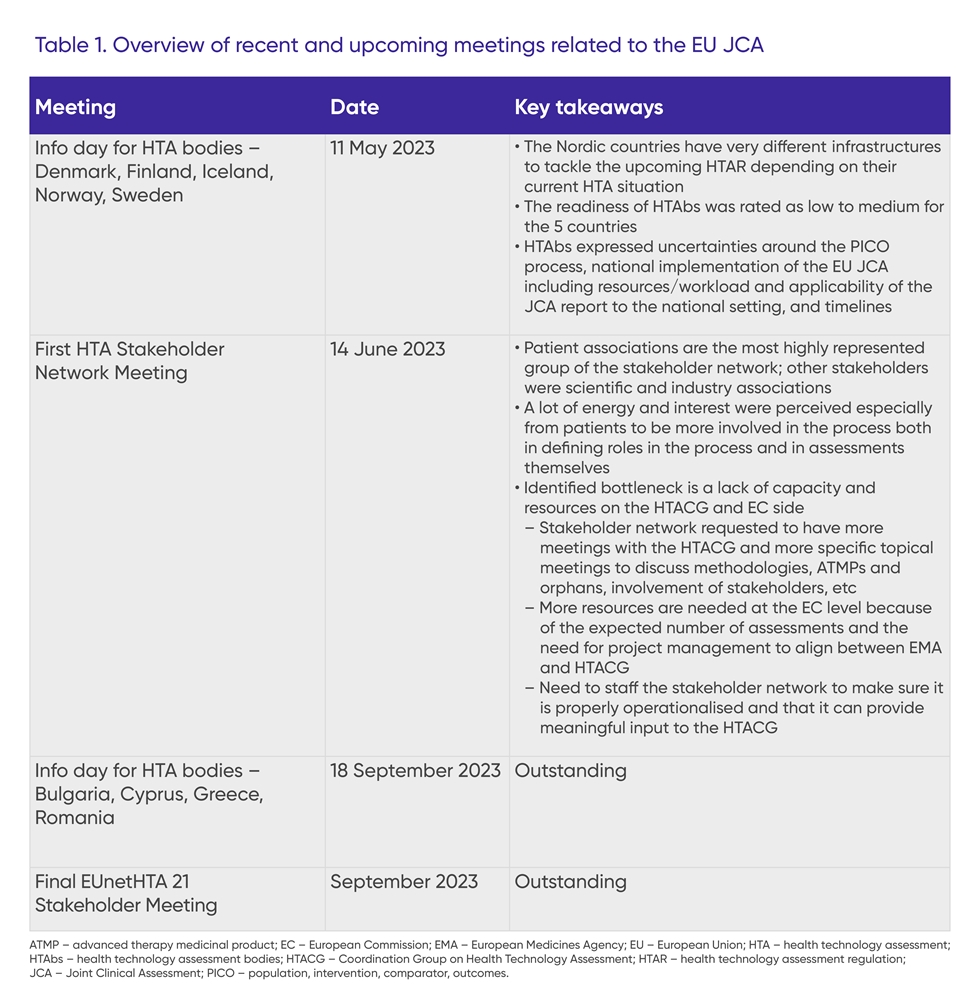
Additionally, the first information day for HTA bodies from the Nordic countries (Denmark, Finland, Iceland, Norway, Sweden) took place in May 2023, and the first HTA Stakeholder Network meeting was held in June 2023. Key takeaways from these meetings and an overview of upcoming meetings are shown in Table 1.
Scoping process
The PICO component of the JCA is one of the items getting the most attention from biopharma companies. EUnetHTA 21 has published a practical guideline, detailing how the scoping process, during which the PICOs are specified, is planned to work (see also Figure 1).
Figure 1. Scoping process
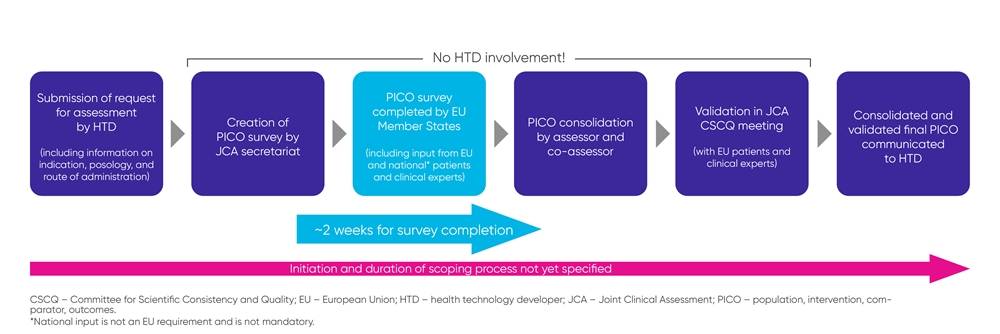
While the scoping process has been defined, there remains a lack of clarity around the comprehensiveness and breadth of the PICO criteria, considering input from the 27 EU member states, and whether consolidated PICOs will adequately represent requirements from the different healthcare systems and treatment guidelines.
It is important to note that biopharma companies will not be involved during the scoping process, which makes early JSC during the clinical trial planning phase especially important to align the design of phase 3 studies with the JCA requirements.
Dossier development
The scoping process is followed by the development of the JCA submission dossier, in which the biopharma company should address all PICO questions identified in the scoping process. While it has been defined that the dossier needs to be submitted at least 45 days prior to the CHMP opinion for medicinal products, the exact initiation date and duration of the scoping process is not yet finalized. Draft timelines published in June 2023 based on a standard average regulatory EMA process distinguished between 2 scenarios (Figure 2):
- (1) new chemical entities; and
- (2) a new indication for an already approved medicinal product (type II variation) with an available JCA report or accelerated regulatory assessment procedures (for medicinal products of major therapeutic interest) for new chemical entities.
For scenario (1), the start of the JCA process is planned approximately 2 months after submission of the regulatory dossier to the EMA, while in scenario (2) the JCA process would start at the time of submission to the EMA. In the event of major deviations in the regulatory process (eg, disconnect between the anticipated and approved indication, longer clock-stop durations), the JCA process must be adapted as well. Draft timelines have been published detailing the important milestones with accompanying dates for both scenarios. Looking at the 2 defined scenarios, the submitting biopharma company will have around 3 months to prepare the dossier. Timelines for medical devices will be developed at a later stage.
Figure 2. Standard average regulatory EMA process scenarios

As with a national HTA submission, dossier development could be started more than 3 months prior to JCA submission, although the benefits of an early start must be balanced against the uncertainty of the request before the PICOs are finalized, particularly for early JCAs when the process is new for all stakeholders. With these short timelines for dossier preparation, biopharma companies should strengthen cross-functional communication between global and local teams to anticipate PICO requests and act on them in a timely manner.
What is still uncertain and how can biopharma companies prepare?
Outstanding deliverables will be finalized based on learnings during the pilot phase; for example, the JSC briefing book template and procedural guidance are due to be finalized by 29 September 2023. An update on the progress of these deliverables can be found at https://www.eunethta.eu/jointhtawork/.
As outlined above, crucial JSC and JCA process guidelines still need to be finalized by EUnetHTA 21, and more thorough involvement of and interaction between all stakeholders (HTA bodies, biopharma companies, Stakeholder Network, HTACG, and the European Commission [EC]) is needed to provide more clarity on the process and prepare all stakeholders properly for the JCA, with implementation only 17 months away.
It remains uncertain whether the JCA will achieve the broader objectives of the regulation, such as faster patient access and reduction of inequalities and bureaucracy. Time to access should theoretically be shortened since the JCA report will be published, at most, 30 days after the EC decision; however, it is still unclear which additional requirements will be requested on a national level. It is the responsibility of all stakeholders to make JCA work to ultimately reduce time to access for patients; while national HTA bodies need to utilize the JCA results, at the same time, biopharma companies also need to comply with evidence requirements to streamline both the JCA and national processes.
Currently, the German HTA bodies G-BA and Institute for Quality and Efficiency in Healthcare (IQWiG), the Dutch Zorginstituut Nederland (ZIN) and the French Haute Autorité de Santé (HAS) are highly involved in the process, and HAS is reportedly working on national implementation of the European HTAR, preparing a department to work at the EU level and adapting national procedures to take the JCA into account. It will be challenging especially for HTA bodies in smaller countries to establish the capacity to shape and support the new JCA process and enable fast development of JCA reports, potentially leading to poor engagement with the process and limited recognition of the results of the report, resulting in subsequent delays in market access.
From the biopharma company perspective, preparation should start as soon as possible to educate internal teams and functions on the JCA processes. PICO discussions will need to take place earlier in the development/regulatory and market access pathways, which has implications for research and development (R&D) and clinical operations departments planning clinical trials based on anticipated PICOs. Cross-functional collaboration between R&D, commercial, and (global) market access teams is critical for success, requiring dedicated resources and deep understanding to enable the development of EMA and JCA dossiers in parallel. Internal and external stakeholder engagement is crucial to best prepare for the JCA process to come.
To conclude, early interaction between all stakeholders and a clear understanding of the process, timelines, and evidence requirements will be crucial contributors to achieving faster market access and availability to patients across Europe and making the EU JCA a success.

Keep up on key global health technology assessment news
HTA Quarterly
Each edition of HTA Quarterly provides insights and analysis from global experts with local market expertise on key priorities and perspectives affecting HTA.
From therapeutic area spotlights to geographic reviews to critical industry topics, keep current on HTA activity around the globe with HTA Quarterly.
Sources
- AmerisourceBergen. The EU JCA process: Opportunities and challenges in bringing innovative medicines to patients in Europe. 21 June 2023. Accessed 11 July 2023. https://www.amerisourcebergen.com/manufacturer-solutions/eu-jca-june-22-webinar-thank-you
- Drummond M, Mittendorf T. The new EU regulation on health technology assessment: Major change or minor adjustment? 31 August 2022. Accessed 1 June 2023. https://www.xcenda.com/insights/htaq-fall-2022-eu-regulation-and-health-technology-assessment
- EUnetHTA. Practical Guideline – D4.2 Scoping Process. Version 1.0, 12 September 2022. https://www.eunethta.eu/wp-content/uploads/2022/09/EUnetHTA-21-D4.2-practical-guideline-on-scoping-process-v1.0.pdf
- EUnetHTA. The EUnetHTA 21 Consortium will cease operations on 16 September 2023. 17 May 2023. Accessed 1 June 2023. https://www.eunethta.eu/the-eunethta-21-consortium-will-cease-operations-on-16-september-2023/
- EUnetHTA. EUnetHTA 21 – Stakeholder Meeting. 12 May 2023. Accessed 1 June 2023. https://www.eunethta.eu/wp-content/uploads/2023/05/EUnetHTA-21-Stakeholder-Meeting-12.05.2023.pdf.
- EUnetHTA. JCA timelines. Version 0.1, 15 March 2023. Accessed 1 June 2023. https://www.eunethta.eu/wp-content/uploads/2023/06/EUnetHTA-21-D5.4-Timelines-JCA.pdf
- European Commission. Health Technology Assessment Stakeholder Network – list of members. Accessed 27 June 2023. https://health.ec.europa.eu/latest-updates/health-technology-assessment-stakeholder-network-list-members-2023-05-05_en
- European Commission. Regulation on Health Technology Assessment. Accessed 27 June 2023. https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment_en
- G-BA. EU-HTA: G-BA coordinates parallel scientific advice for manufcturer from September onwards. 3 July 2023. Accessed 10 July 2023. https://www.g-ba.de/presse/pressemitteilungen-meldungen/1118/
- Kulp W, Martel MJ, Billig S, et al. Joint clinical assessment in the EU: Pan-European HTA for drugs and medical devices will become reality. 17 February 2022. Accessed 1 June 2023. https://www.xcenda.com/insights/htaq-spring-2022-joint-clinical-assessment-eu




