The Cost of Cures: What Does Zolgensma Teach Us About the Use of Cost-Effectiveness Assessments?
By Xcenda
Earlier this year, the US FDA approved the first gene therapy to treat the most severe form of spinal muscular atrophy, ZOLGENSMA (onasemnogene abeparvovec). However, a lot of attention was focused on the price tag of the therapy. With million-dollar cures coming to market, what are the current perceptions of value and the role of cost-effectiveness? In this article, we explore how and when cost-effectiveness assessments are utilized for making reimbursement and pricing decisions across several major markets.
HTA QUARTERLY | FALL 2019
The Cost of Cures: What Does Zolgensma Teach Us About the Use of Cost-Effectiveness Assessments?
Introduction
On May 24, 2019, the United States (US) Food and Drug Administration (FDA) approved ZOLGENSMA (onasemnogene abeparvovec), a 1-time treatment for spinal muscular atrophy (SMA) and the first gene therapy approved to treat the most severe form of the disease. Although it was hailed as a medical breakthrough, the headlines that proliferated in the media were not confined to the enormous benefits the therapy brings to pediatric patients diagnosed with a rare, and often fatal, genetic illness. Rather, the attention was primarily on the US$2.125 million price tag, which currently represents the world’s most expensive medicine. Many criticized Novartis, the drug’s manufacturer, for the “unimaginably high price,” while others have argued that the advantages of ZOLGENSMA go beyond the eye-popping figure to represent a crucial milestone for patients who suffer from SMA. Now that we are in an era where million-dollar cures are emerging, what can the ZOLGENSMA example teach us about the current perceptions of value and the role of cost-effectiveness (CE) analyses? In this article, we explore how and when CE assessments are utilized for making reimbursement and pricing decisions across several major markets.
Insights From the US’s Unofficial Health Technology Assessment Organization
Although the US healthcare system has historically rejected the use of CE to drive access and favored choice over equality, the environment is changing. Prior to ZOLGENSMA’s approval, on April 3, 2019, the Institute for Clinical and Economic Review (the Institute), the unofficial drug pricing watchdog in the US, released the Final Evidence Report for its assessment of SMA treatments, including both SPINRAZA (nusinersen) and ZOLGENSMA. During the meeting of the New England Comparative Effectiveness Public Advisory Council (CEPAC), one of the Institute’s 3 independent evidence appraisal committees, the panel voted unanimously that although the clinical evidence was sufficient to show a net health benefit for both drugs, the price for SPINRAZA, which was approved in December of 2016, is too high to align fairly with these benefits and, at the same time, encouraged Novartis to set a lower price for ZOLGENSMA once it entered the market. Shortly following ZOLGENSMA’s FDA approval in May, the Institute announced the release of an addendum stating that in light of the labeled indication and newly available efficacy data, ZOLGENSMA’s list price of $2.125 million falls within the upper bound of the organization’s CE threshold range of $100,000 to 150,000 per life-year gained (LYG). As part of the current iteration of the Institute’s Value Assessment Framework (VAF), the cost per LYG metric represents an additional measure of clinical benefit and is presented along with the cost per quality-adjusted life-year (QALY) in every assessment in order to increase transparency around the incremental cost-effectiveness ratio (ICER) calculations. As ZOLGENSMA would have not otherwise been considered cost-effective (that is, fallen below the Institute’s prespecified limit of $150,000 per QALY), this example represents the importance of going beyond traditional cost-effectiveness analyses (CEAs), particularly for curative gene therapies, by including different perspectives of product value to advance patient access to groundbreaking treatments.
Use of CEAs in Global Markets
In markets outside the US, health technology assessment (HTA) organizations commonly use a prespecified CE threshold range for making reimbursement decisions; these ranges may be explicit or implicit. Nevertheless, some do not place a specific limit on cost-effectiveness to determine a product’s value. An overview of the use of CE thresholds in several countries is shown in Table 1.
Table 1. Overview of CE Thresholds
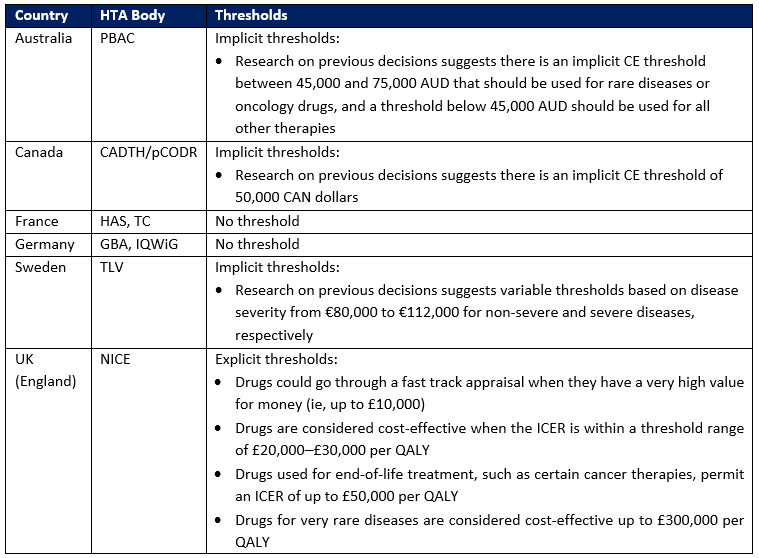
Key: CADTH – Canadian Agency for Drugs and Technologies in Health; GBA – Federal Joint Committee; HAS – Haute Autorité de Santé; IQWiG – Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen); NICE – National Institute for Health and Care Excellence; PBAC – Pharmaceutical Benefits Advisory Committee; pCODR – pan-Canadian Oncology Drug Review; TC – Transparency Committee (Commission de la Transparence, CT); TLV – Dental and Pharmaceutical Benefits Agency (Tandvårds-Läkemedelförmånsverket).
Although SPINRAZA, a competitor to ZOLGENSMA, was approved in Europe in 2017, the National Institute for Health and Care Excellence (NICE) did not recommend its use through the National Health Service (NHS) in England until 2019 after reaching a price agreement at a discounted rate. ZOLGENSMA has not been approved by the European Medicines Agency or evaluated by HTA agencies outside of the US, but many key opinion leaders believe it will confront similar pricing and reimbursement discussions. Thus, CEAs will likely play an important role in making reimbursement decisions for high-cost gene therapies.
Challenges in the Use of CEAs for Gene Therapies
Despite criticism, healthcare decision makers in the UK, Canada, and Australia view the QALY as the best tool to consistently and objectively assess the value of therapies across indications. However, even in markets that utilize CE analyses and the QALY for reimbursement, the differences in their application highlight inherent uncertainties and controversies.
For example, while NICE uses a CE threshold range to make reimbursement decisions, the Pharmaceutical Benefits Advisory Committee (PBAC) of Australia and the Canadian Agency for Drugs and Technologies in Health (CADTH) do not explicitly or consistently use such benchmarks. It has been argued that NICE’s use of CE thresholds may incentivize manufacturers to target their economic analysis to achieve a certain ICER. In a recent study, Wang and colleagues compared the ICERs in 58 drug reimbursement submissions to NICE and PBAC to determine whether certain CE thresholds affected the proposed ICER, and they found significantly higher ICERs in NICE submissions relative to PBAC.
Challenges also exist with the use of higher thresholds for expensive end-of-life, cancer, and rare disease therapies, which often require additional evidence to demonstrate that a product is cost-effective. In England, NICE gives special consideration to end-of-life treatments and very rare diseases by applying higher explicit thresholds, and the Patented Medicine Prices Review Board (PMPRB) of Canada has recently indicated that it will consider a higher CE threshold for rare disease treatments. Furthermore, the use of CE thresholds does not account for short-term budget impact and potential opportunity costs. With new, very expensive innovative treatments like ZOLGENSMA entering the market, there might be a need for another metric to determine a drug’s value. Constrained resources often dictate the use of funding for one technology over another, so decision makers need to be able to identify the benefits that might be gained by accelerating access to life-extending treatments.
Prepare for Assessment With Early Economic Models
In the current environment in which health technology appraisals and CEAs drive reimbursement, pricing, and access, demonstrating the economic value of new therapies is critical. To prepare for these appraisals, manufacturers can utilize economic models to assess the value of their assets. Early economic models are typically designed to emulate future assessments, with CEAs being most common, but they can be adopted to fit the unique characteristics and needs of a particular therapy. They enable planning for various clinical and pricing scenarios and answer important questions like:
- What is the therapy’s potential clinical, economic, and humanistic value?
- What is the therapy’s potential value-based price?
- What are the gaps in the existing literature?
- What are the implications of different choices in the clinical program?
By designing an adoptable and dynamic model, users can assess the implications of different choices in context of a specific clinical program, such as the target patient population, place in therapy, and clinical outcome assessment selection. In addition to the model outputs, including the potential value-based price, the development process itself often highlights the key value drivers for the therapy and helps manufacturers identify the gaps in the existing evidence base. Thus, it is important to engage in model development as early as possible to inform critical go/no-go decisions.
Conclusion
CEAs play an important role in reimbursement and pricing decisions in many countries across the world, and their application in the US is growing. Despite criticism, the QALY remains the gold standard for health economists to assess the value of treatments and is likely to remain a critical appraisal tool for many years to come. However, the evaluation of ZOLGENSMA by the Institute for Clinical and Economic Review demonstrates the importance and value of utilizing other measures of cost-effectiveness. Manufacturers should embrace these evidentiary requirements and prepare to demonstrate the value of their products in the early stages of clinical development. The need to prepare for value assessments is especially evident for potential cures of ultra-rare conditions like SMA, and early economic models are a vital resource to that end.
The article should be referenced as follows:
Loos A, van Stiphout J, Campbell D. The cost of cures: what does Zolgensma teach us about the use of cost-effectiveness assessments? HTA Quarterly. Fall 2019. https://www.xcenda.com/insights/htaq-fall-2019-the-cost-of-cures-zolgensma-cea.
References
- Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016 Dec 1;94(12):925-930.
- FDA news release. FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. May 24, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease.
- Forbes. At over $2 million Zolgensma is the world's most expensive therapy, yet relatively cost-effective. June 5, 2019. https://www.forbes.com/sites/joshuacohen/2019/06/05/at-over-2-million-zolgensma-is-the-worlds-most-expensive-therapy-yet-relatively-cost-effective/#697e9b9945f5.
- ICER. ICER final evidence report. Spinraza® and Zolgensma® for spinal muscular atrophy: effectiveness and value. April 3, 2019. https://icer-review.org/wp-content/uploads/2018/07/ICER_SMA_Final_Evidence_Report_052419.pdf.
- ICER. ICER comments on the FDA approval of Zolgensma for the treatment of spinal muscular atrophy. May 24, 2019. https://icer-review.org/announcements/icer_comment_on_zolgensma_approval/.
- ICER. ICER seeks public input for 2020 value assessment framework. May 2, 2019. https://icer-review.org/announcements/icer-seeks-public-input-for-2020-value-assessment-framework/.
- Martell A. Canadian drug price regulator may be flexible on rare diseases. https://www.reuters.com/article/us-canada-pharmaceuticals-rare/canadian-drug-price-regulator-may-be-flexible-on-rare-diseases-idUSKCN1V31JN.
- NICE. Guide to the methods of technology appraisal 2013, April 2013. https://www.nice.org.uk/process/pmg9/chapter/the-appraisal-of-the-evidence-and-structured-decision-making.
- NICE. Consultation paper value based assessment of health technologies. https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/VBA-TA-Methods-Guide-for-Consultation.pdf.
- Paris V, Belloni A. Value in pharmaceutical pricing country profile: Australia. OECD 2014. https://www.oecd.org/health/Value-in-Pharmaceutical-Pricing-Australia.pdf.
- Paulen M. Recent amendments to NICE's value-based assessment of health technologies: implicitly inequitable? Expert Rev Pharmacoecon Outcomes Res. 2017 Jun;17(3):239-242.
- Pharmaceutical Technology. Zolgensma’s reimbursement barriers represent an uphill task for gene therapy developers. July 22, 2019. https://www.pharmaceutical-technology.com/comment/zolgensma-cost/.
- Svensson M, Nilsson F, Arnberg K. Reimbursement decisions for pharmaceuticals in Sweden: the impact of disease severity and cost effectiveness. Pharmacoeconomics. 2015 Nov;33(11):1229.
- The Washington Post. High fives and sobs greet UnitedHealthcare’s reversal of denials for gene therapy. July 18, 2019. https://www.washingtonpost.com/business/economy/high-fives-and-sobs-greet-unitedhealthcares-reversal-of-denials-for-child-gene-therapy/2019/07/18/8ddeb3ae-a974-11e9-9214-246e594de5d5_story.html?noredirect=on&utm_term=.9b69aa2e7fee.
- STAT news. ‘Anchoring’ was at work in setting the price of Novartis’ new gene therapy. June 4, 2019. https://www.statnews.com/2019/06/04/anchoring-price-zolgensma/.
- STAT news. I have spinal muscular atrophy. Critics of the $2 million new gene therapy are missing the point. https://www.statnews.com/2019/05/31/spinal-muscular-atrophy-zolgensma-price-critics/.
- Wang S, Gum D, Merlin T. Comparing the ICERs in medicine reimbursement submissions to NICE and PBAC – does the presence of an explicit threshold affect the ICER proposed? Value Health. 2018;21(8):938-43.



